Allotrope Of Carbon on:
[Wikipedia]
[Google]
[Amazon]


 Carbon nanobuds are a newly discovered allotrope of
Carbon nanobuds are a newly discovered allotrope of
 Glassy carbon or vitreous carbon is a class of non-graphitizing
Glassy carbon or vitreous carbon is a class of non-graphitizing
 bcc-carbon: At ultrahigh pressures of above 1000 GPa, diamond is predicted to transform into a
bcc-carbon: At ultrahigh pressures of above 1000 GPa, diamond is predicted to transform into a  * The
* The
 The system of carbon allotropes spans an astounding range of extremes, considering that they are all merely structural formations of the same element.
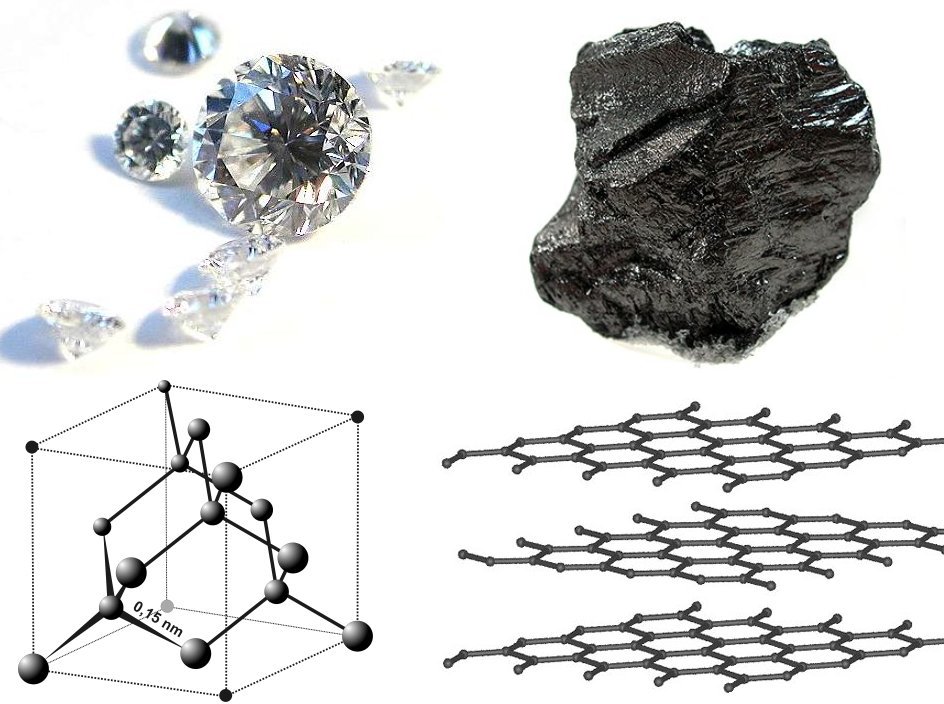
Between diamond and graphite:
* Diamond crystallizes in the cubic system but graphite crystallizes in the hexagonal system.
* Diamond is clear and transparent, but graphite is black and opaque.
* Diamond is the hardest mineral known (10 on the
The system of carbon allotropes spans an astounding range of extremes, considering that they are all merely structural formations of the same element.
Between diamond and graphite:
* Diamond crystallizes in the cubic system but graphite crystallizes in the hexagonal system.
* Diamond is clear and transparent, but graphite is black and opaque.
* Diamond is the hardest mineral known (10 on the

Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
is capable of forming many allotropes
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: th ...
(structurally different forms of the same element) due to its valency. Well-known forms of carbon include diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the Chemical stability, chemically stable form of car ...
and graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large ...
. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene
Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a soccer ball. Each of its 60 carbon atoms is bonded ...
and sheets such as graphene
Graphene () is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure.
. Larger-scale structures of carbon include carbon nano tube, nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA).
Diamond
Diamond is a well-known allotrope of carbon. Thehardness
In materials science, hardness (antonym: softness) is a measure of the resistance to localized plastic deformation induced by either mechanical indentation or abrasion. In general, different materials differ in their hardness; for example hard ...
, extremely high refractive index
In optics, the refractive index (or refraction index) of an optical medium is a dimensionless number that gives the indication of the light bending ability of that medium.
The refractive index determines how much the path of light is bent, or ...
, and high dispersion of light make diamond useful for industrial applications and for jewelry. Diamond is the hardest known natural mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. ( ...
. This makes it an excellent abrasive and makes it hold polish and luster extremely well. No known naturally occurring substance can cut or scratch diamond, except another diamond. In diamond form, carbon is one of the costliest elements.
The crystal structure of diamond is a face-centred cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
lattice having eight atoms per unit cell to form a diamond cubic
The diamond cubic crystal structure is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was diamond, other elements in group 14 also adopt this structure, including α-tin, the sem ...
structure. Each carbon atom is covalently bonded
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
to four other carbons in a tetrahedral geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1(−) = 109.4712206...° ≈ 109.5° when all four substituents are ...
. These tetrahedrons together form a 3-dimensional network of six-membered carbon rings in the chair conformation
In organic chemistry, cyclohexane conformations are any of several three-dimensional shapes adopted by molecules of cyclohexane. Because many compounds feature structurally similar six-membered rings, the structure and dynamics of cyclohexane are ...
, allowing for zero bond angle
Bond or bonds may refer to:
Common meanings
* Bond (finance), a type of debt security
* Bail bond, a commercial third-party guarantor of surety bonds in the United States
* Chemical bond, the attraction of atoms, ions or molecules to form chemical ...
strain. The bonding occurs through sp3 hybridized orbitals to give a C-C bond length of 154 pm. This network of unstrained covalent bonds makes diamond extremely strong. Diamond is thermodynamically less stable than graphite at pressures below .
The dominant industrial use of diamond is cutting
Cutting is the separation or opening of a physical object, into two or more portions, through the application of an acutely directed force.
Implements commonly used for wikt:cut, cutting are the knife and saw, or in medicine and science the scal ...
, drilling
Drilling is a cutting process where a drill bit is spun to cut a hole of circular cross-section in solid materials. The drill bit is usually a rotary cutting tool, often multi-point. The bit is pressed against the work-piece and rotated at ra ...
(drill bit
Drill bits are cutting tools used in a drill to remove material to create holes, almost always of circular cross-section. Drill bits come in many sizes and shapes and can create different kinds of holes in many different materials. In order ...
s), grinding
Grind is the cross-sectional shape of a blade.
Grind, grinds, or grinding may also refer to:
Grinding action
* Grinding (abrasive cutting), a method of crafting
* Grinding (dance), suggestive club dancing
* Grinding (video gaming), repetitive and ...
(diamond edged cutters), and polishing. Most uses of diamonds in these technologies do not require large diamonds, and most diamonds that are not gem-quality can find an industrial use. Diamonds are embedded in drill tips and saw blades, or ground into a powder for use in grinding and polishing applications (due to its extraordinary hardness). Specialized applications include use in laboratories as containment for high pressure experiments (see diamond anvil
A diamond anvil cell (DAC) is a high-pressure device used in geology, engineering, and materials science experiments. It enables the compression of a small (sub-millimeter-sized) piece of material to extreme pressures, typically up to around 10 ...
), high-performance bearings, and specialized window
A window is an opening in a wall, door, roof, or vehicle that allows the exchange of light and may also allow the passage of sound and sometimes air. Modern windows are usually glazed or covered in some other transparent or translucent materia ...
s of technical apparatuses.
The market for industrial-grade diamonds operates much differently from its gem-grade counterpart. Industrial diamonds are valued mostly for their hardness and heat conductivity, making many of the gemological
Gemology or gemmology is the science dealing with natural and artificial gemstone materials. It is a geoscience and a branch of mineralogy. Some jewelers (and many non-jewelers) are academically trained gemologists and are qualified to identify ...
characteristics of diamond, including clarity and color, mostly irrelevant. This helps explain why 80% of mined diamonds (equal to about 100 million carats or 20 tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
s annually) are unsuitable for use as gemstones and known as ''bort
Bort, boart, or boort is an umbrella term used in the diamond industry to refer to shards of non-gem-grade/quality diamonds. In the manufacturing and heavy industries, "bort" is used to describe dark, imperfectly formed or crystallized diamonds ...
'', are destined for industrial use. In addition to mined diamonds, synthetic diamond
Lab-grown diamond (LGD; also called laboratory-grown, laboratory-created, man-made, artisan-created, artificial, synthetic, or cultured diamond) is diamond that is produced in a controlled technological process (in contrast to naturally formed ...
s found industrial applications almost immediately after their invention in the 1950s; another 400 million carats (80 tonnes) of synthetic diamonds are produced annually for industrial use, which is nearly four times the mass of natural diamonds mined over the same period.
With the continuing advances being made in the production of synthetic diamond, future applications are beginning to become feasible. Garnering much excitement is the possible use of diamond as a semiconductor
A semiconductor is a material which has an electrical resistivity and conductivity, electrical conductivity value falling between that of a electrical conductor, conductor, such as copper, and an insulator (electricity), insulator, such as glas ...
suitable to build microchip
An integrated circuit or monolithic integrated circuit (also referred to as an IC, a chip, or a microchip) is a set of electronic circuits on one small flat piece (or "chip") of semiconductor material, usually silicon. Large numbers of tiny ...
s from, or the use of diamond as a heat sink
A heat sink (also commonly spelled heatsink) is a passive heat exchanger that transfers the heat generated by an electronic or a mechanical device to a fluid medium, often air or a liquid coolant, where it is dissipated away from the device, th ...
in electronics
The field of electronics is a branch of physics and electrical engineering that deals with the emission, behaviour and effects of electrons using electronic devices. Electronics uses active devices to control electron flow by amplification ...
. Significant research efforts in Japan
Japan ( ja, 日本, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the north ...
, Europe
Europe is a large peninsula conventionally considered a continent in its own right because of its great physical size and the weight of its history and traditions. Europe is also considered a Continent#Subcontinents, subcontinent of Eurasia ...
, and the United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territorie ...
are under way to capitalize on the potential offered by diamond's unique material properties, combined with increased quality and quantity of supply starting to become available from synthetic diamond manufacturers.
Graphite
Graphite, named byAbraham Gottlob Werner
Abraham Gottlob Werner (; 25 September 174930 June 1817) was a German geologist who set out an early theory about the stratification of the Earth's crust and propounded a history of the Earth that came to be known as Neptunism. While most tenet ...
in 1789, from the Greek γράφειν (, "to draw/write", for its use in pencils) is one of the most common allotropes of carbon. Unlike diamond, graphite is an electrical conductor. Thus, it can be used in, for instance, electrical arc lamp electrodes. Likewise, under standard conditions
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union ...
, graphite is the most stable form of carbon. Therefore, it is used in thermochemistry as the standard state
In chemistry, the standard state of a material (pure substance, mixture or solution) is a reference point used to calculate its properties under different conditions. A superscript circle ° (degree symbol) or a Plimsoll (⦵) character is use ...
for defining the heat of formation
In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state, wit ...
of carbon compounds.
Graphite conducts electricity, due to delocalization
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly diff ...
of the pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals ...
electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s above and below the planes of the carbon atoms. These electrons are free to move, so are able to conduct electricity. However, the electricity is only conducted along the plane of the layers. In diamond, all four outer electrons of each carbon atom are 'localized' between the atoms in covalent bonding. The movement of electrons is restricted and diamond does not conduct an electric current. In graphite, each carbon atom uses only 3 of its 4 outer energy level electrons in covalently bonding to three other carbon atoms in a plane. Each carbon atom contributes one electron to a delocalized system of electrons that is also a part of the chemical bonding. The delocalized electrons are free to move throughout the plane. For this reason, graphite conducts electricity along the planes of carbon atoms, but does not conduct electricity in a direction at right angles to the plane.
Graphite powder is used as a dry lubricant
A lubricant (sometimes shortened to lube) is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, t ...
. Although it might be thought that this industrially important property is due entirely to the loose interlamellar coupling between sheets in the structure, in fact in a vacuum
A vacuum is a space devoid of matter. The word is derived from the Latin adjective ''vacuus'' for "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often dis ...
environment (such as in technologies for use in space
Space is the boundless three-dimensional extent in which objects and events have relative position and direction. In classical physics, physical space is often conceived in three linear dimensions, although modern physicists usually consider ...
), graphite was found to be a very poor lubricant. This fact led to the discovery that graphite's lubricity is due to adsorbed
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a ...
air and water between the layers, unlike other layered dry lubricants such as molybdenum disulfide
Molybdenum disulfide (or moly) is an inorganic compound composed of molybdenum and sulfur. Its chemical formula is .
The compound is classified as a transition metal dichalcogenide. It is a silvery black solid that occurs as the mineral molybdenit ...
. Recent studies suggest that an effect called superlubricity
In physics (specifically tribology), superlubricity is a regime of motion in which friction vanishes or very nearly vanishes. What is a "vanishing" friction level is not clear, which makes the term quite vague. As an ''ad hoc'' definition, a ki ...
can also account for this effect.
When a large number of crystallographic defects (physical) bind these planes together, graphite loses its lubrication properties and becomes pyrolytic carbon
Pyrolytic carbon is a material similar to graphite, but with some covalent bonding between its graphene sheets as a result of imperfections in its production.
Pyrolytic carbon is man-made and is thought not to be found in nature.Ratner, Buddy D. ...
, a useful material in blood-contacting implants such as prosthetic
In medicine, a prosthesis (plural: prostheses; from grc, πρόσθεσις, prósthesis, addition, application, attachment), or a prosthetic implant, is an artificial device that replaces a missing body part, which may be lost through trau ...
heart valve
A heart valve is a one-way valve that allows blood to flow in one direction through the chambers of the heart. Four valves are usually present in a mammalian heart and together they determine the pathway of blood flow through the heart. A heart v ...
s.
Graphite is the most stable allotrope of carbon. Contrary to popular belief, high-purity graphite does not readily burn, even at elevated temperatures. For this reason, it is used in nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nu ...
s and for high-temperature crucibles for melting metals. At very high temperatures and pressures (roughly 2000 °C and 5 GPa), it can be transformed into diamond.
Natural and crystalline graphites are not often used in pure form as structural materials due to their shear-planes, brittleness and inconsistent mechanical properties.
In its pure glassy (isotropic) synthetic forms, pyrolytic graphite
Pyrolytic carbon is a material similar to graphite, but with some covalent bonding between its graphene sheets as a result of imperfections in its production.
Pyrolytic carbon is man-made and is thought not to be found in nature.Ratner, Buddy D. ...
and carbon fiber
Carbon fiber-reinforced polymers (American English), carbon-fibre-reinforced polymers (Commonwealth English), carbon-fiber-reinforced plastics, carbon-fiber reinforced-thermoplastic (CFRP, CRP, CFRTP), also known as carbon fiber, carbon compo ...
graphite are extremely strong, heat-resistant (to 3000 °C) materials, used in reentry shields for missile nosecones, solid rocket
A solid-propellant rocket or solid rocket is a rocket with a rocket engine that uses solid propellants
Rocket propellant is the reaction mass of a rocket. This reaction mass is ejected at the highest achievable velocity from a rocket engine ...
engines, high temperature reactors, brake
A brake is a mechanical device that inhibits motion by absorbing energy from a moving system. It is used for slowing or stopping a moving vehicle, wheel, axle, or to prevent its motion, most often accomplished by means of friction.
Background ...
shoes and electric motor
An electric motor is an Electric machine, electrical machine that converts electrical energy into mechanical energy. Most electric motors operate through the interaction between the motor's magnetic field and electric current in a Electromagneti ...
brushes
A brush is a common tool with bristles, wire or other filaments. It generally consists of a handle or block to which filaments are affixed in either a parallel or perpendicular orientation, depending on the way the brush is to be gripped durin ...
.
Intumescent or expandable graphites are used in fire seals, fitted around the perimeter of a fire door. During a fire the graphite intumesces (expands and chars) to resist fire penetration and prevent the spread of fumes. A typical ''start expansion temperature'' (SET) is between 150 and 300 °C.
Density: Graphite's specific gravity is 2.3, which makes it lighter than diamond.
Chemical activity: it is slightly more reactive than diamond. This is because the reactants are able to penetrate between the hexagonal layers of carbon atoms in graphite. It is unaffected by ordinary solvents, dilute acids, or fused alkalis. However, chromic acid
The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixt ...
oxidizes it to carbon dioxide.
Graphene
A single layer of graphite is calledgraphene
Graphene () is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure.
and has extraordinary electrical, thermal, and physical properties. It can be produced by epitaxy
Epitaxy refers to a type of crystal growth or material deposition in which new crystalline layers are formed with one or more well-defined orientations with respect to the crystalline seed layer. The deposited crystalline film is called an epit ...
on an insulating or conducting substrate or by mechanical exfoliation (repeated peeling) from graphite. Its applications may include replacing silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic tab ...
in high-performance electronic devices. With two layers stacked, bilayer graphene
Bilayer graphene is a material consisting of two layers of graphene. One of the first reports of bilayer graphene was in the seminal 2004 '' Science (journal), Science'' paper by Geim and colleagues, in which they described devices "which containe ...
results with different properties.
Lonsdaleite (hexagonal diamond)
Lonsdaleite
Lonsdaleite (named in honour of Kathleen Lonsdale), also called hexagonal diamond in reference to the crystal structure, is an allotrope of carbon with a hexagonal lattice, as opposed to the cubical lattice of conventional diamond. It is found i ...
is an allotrope sometimes called "hexagonal
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A '' regular hexagon'' has ...
diamond", formed from graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large ...
present in meteor
A meteoroid () is a small rocky or metallic body in outer space.
Meteoroids are defined as objects significantly smaller than asteroids, ranging in size from grains to objects up to a meter wide. Objects smaller than this are classified as micr ...
ites upon their impact on the earth. The great heat and pressure of the impact transforms the graphite into a denser form similar to diamond, but retaining graphite's hexagonal crystal lattice
In geometry and crystallography, a Bravais lattice, named after , is an infinite array of discrete points generated by a set of discrete translation operations described in three dimensional space by
: \mathbf = n_1 \mathbf_1 + n_2 \mathbf_2 + n ...
. "Hexagonal diamond" has also been synthesized in the laboratory, by compressing and heating graphite either in a static press or using explosives. It can also be produced by the thermal decomposition of a polymer, poly(hydridocarbyne), at atmospheric pressure, under inert gas atmosphere (e.g. argon, nitrogen), starting at temperature .
Graphenylene
Graphenylene is a single layer carbon material withbiphenylene
Biphenylene is an organic compound with the formula (C6H4)2. It is a pale, yellowish solid with a hay-like odor. Despite its unusual structure, it behaves like a traditional polycyclic aromatic hydrocarbon.
Bonding
Biphenylene is a polycyclic h ...
-like subunits as basis in its hexagonal lattice structure. It is also known as biphenylene-carbon.
Carbophene
Carbophene is a 2 dimensionalcovalent organic framework
Covalent organic frameworks (COFs) are a class of materials that form two- or three-dimensional structures through reactions between organic precursors resulting in strong, covalent bonds to afford porous, stable, and crystalline materials. COFs em ...
. 4-6 carbophene has been synthesized from 1-3-5 trihydroxybenzene. It consists of 4-carbon and 6-carbon rings in 1:1 ratio. The angles between the three σ-bonds of the orbitals are approximately 120°, 90°, and 150°.
AA'-graphite
AA'-graphite is an allotrope of carbon similar to graphite, but where the layers are positioned differently to each other as compared to the order in graphite.Diamane
Diamane is a 2D form of diamond. It can be made via high pressures, but without that pressure, the material reverts to graphene. Another technique is to add hydrogen atoms but those bonds are weak. Using fluorine (xenon-difluoride) instead brings the layers closer together, strengthening the bonds. This is called f-diamane.Amorphous carbon
Amorphous carbon is the name used forcarbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
that does not have any crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
line structure. As with all glassy materials, some short-range order can be observed, but there is no long-range pattern of atomic positions. While entirely amorphous carbon can be produced, most amorphous carbon actually contains microscopic crystals of graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large ...
-like, or even diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the Chemical stability, chemically stable form of car ...
-like carbon.
Coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal is formed when dea ...
and soot
Soot ( ) is a mass of impure carbon particles resulting from the incomplete combustion of hydrocarbons. It is more properly restricted to the product of the gas-phase combustion process but is commonly extended to include the residual pyrolysed ...
or carbon black
Carbon black (subtypes are acetylene black, channel black, furnace black, lamp black and thermal black) is a material produced by the incomplete combustion of coal and coal tar, vegetable matter, or petroleum products, including fuel oil, fluid ...
are informally called amorphous carbon. However, they are products of pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''py ...
(the process of decomposing a substance by the action of heat), which does not produce true amorphous carbon under normal conditions.
Nanocarbons
Buckminsterfullerenes
The ''buckminsterfullerenes'', or usually just ''fullerenes'' or ''buckyballs'' for short, were discovered in 1985 by a team of scientists from Rice University and the University of Sussex, three of whom were awarded the 1996 Nobel Prize in Chemistry. They are named for the resemblance to the geodesic structures devised by Richard Buckminster "Bucky" Fuller. Fullerenes are positively curved molecules of varying sizes composed entirely of carbon, which take the form of a hollow sphere, ellipsoid, or tube. As of the early twenty-first century, the chemical and physical properties of fullerenes are still under heavy study, in both pure and applied research labs. In April 2003, fullerenes were under study for potential medicinal use — binding specific antibiotics to the structure to target resistant bacteria and even target certain cancer cells such as melanoma.Carbon nanotubes
Carbon nanotubes, also called buckytubes, are cylindricalcarbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
molecules
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
with novel properties that make them potentially useful in a wide variety of applications (e.g., nano-electronics, optics
Optics is the branch of physics that studies the behaviour and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behaviour of visible, ultraviole ...
, materials
Material is a substance or mixture of substances that constitutes an object. Materials can be pure or impure, living or non-living matter. Materials can be classified on the basis of their physical and chemical properties, or on their geologic ...
applications, etc.). They exhibit extraordinary strength, unique electrical
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by ...
properties, and are efficient conductors of heat
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is al ...
. Non-carbon nanotube
A non-carbon nanotube is a cylindrical molecule often composed of metal oxides, or group III-Nitrides and morphologically similar to a carbon nanotube. Non-carbon nanotubes have been observed to occur naturally in some mineral deposits.
A few year ...
s have also been synthesized.
Carbon nanotubes are a members of the fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
structural family, which also includes buckyball
Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a soccer ball. Each of its 60 carbon atoms is bonded t ...
s. Whereas buckyballs are spherical
A sphere () is a geometrical object that is a three-dimensional analogue to a two-dimensional circle. A sphere is the set of points that are all at the same distance from a given point in three-dimensional space.. That given point is the ce ...
in shape, a nanotube is cylindrical
A cylinder (from ) has traditionally been a three-dimensional solid, one of the most basic of curvilinear geometric shapes. In elementary geometry, it is considered a prism with a circle as its base.
A cylinder may also be defined as an infini ...
, with at least one end typically capped with a hemisphere of the buckyball structure. Their name is derived from their size, since the diameter of a nanotube is on the order of a few nanometer
330px, Different lengths as in respect to the molecular scale.
The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm) or nanometer (American and British English spelling differences#-re ...
s (approximately 50,000 times smaller than the width of a human hair), while they can be up to several centimeters in length. There are two main types of nanotubes: single-walled nanotubes (SWNTs) and multi-walled nanotubes (MWNTs).
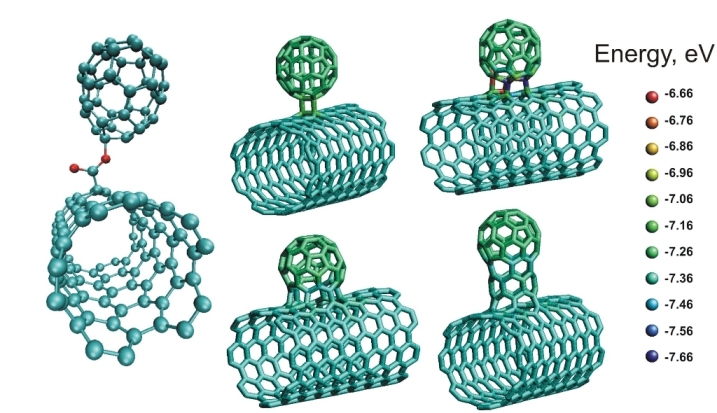
Carbon nanobuds
 Carbon nanobuds are a newly discovered allotrope of
Carbon nanobuds are a newly discovered allotrope of carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
in which fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
like "buds" are covalently attached to the outer sidewalls of the carbon nanotubes
A scanning tunneling microscopy image of a single-walled carbon nanotube
Rotating single-walled zigzag carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with diameters typically measured in nanometers.
''Single-wall carbon nan ...
. This hybrid material has useful properties of both fullerenes and carbon nanotubes. For instance, they have been found to be exceptionally good field emitters.
Schwarzites
Schwarzites are negatively curved carbon surfaces originally proposed by decoratingtriply periodic minimal surface
In differential geometry, a triply periodic minimal surface (TPMS) is a minimal surface in ℝ3 that is invariant under a rank-3 lattice of translations.
These surfaces have the symmetries of a crystallographic group. Numerous examples are know ...
s with carbon atoms. The geometric topology
In mathematics, geometric topology is the study of manifolds and maps between them, particularly embeddings of one manifold into another.
History
Geometric topology as an area distinct from algebraic topology may be said to have originated i ...
of the structure is determined by the presence of ring defects, such as heptagons and octagons, to graphene
Graphene () is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure.
's hexagonal lattice.
(Negative curvature
In mathematics, curvature is any of several strongly related concepts in geometry. Intuitively, the curvature is the amount by which a curve deviates from being a straight line, or a surface deviates from being a plane.
For curves, the canonic ...
bends surfaces outwards like a saddle rather than bending inwards like a sphere.)
Recent work has proposed zeolite-templated carbons (ZTCs) may be schwarzites. The name, ZTC, derives from their origin inside the pores of zeolite
Zeolites are microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a metal ion or H+. These pos ...
s, crystalline silicon dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one ...
minerals. A vapor of carbon-containing molecules is injected into the zeolite, where the carbon gathers on the pores' walls, creating the negative curve. Dissolving the zeolite leaves the carbon. A team generated structures by decorating the pores of a zeolite with carbon through a Monte Carlo method
Monte Carlo methods, or Monte Carlo experiments, are a broad class of computational algorithms that rely on repeated random sampling to obtain numerical results. The underlying concept is to use randomness to solve problems that might be determi ...
. Some of the resulting models resemble schwarzite-like structures.
Glassy carbon
 Glassy carbon or vitreous carbon is a class of non-graphitizing
Glassy carbon or vitreous carbon is a class of non-graphitizing carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
widely used as an electrode material in electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
, as well as for high-temperature crucibles and as a component of some prosthetic devices.
It was first produced by Bernard Redfern in the mid-1950s at the laboratories of The Carborundum Company, Manchester, UK. He had set out to develop a polymer matrix to mirror a diamond structure and discovered a resole (phenolic) resin that would, with special preparation, set without a catalyst. Using this resin the first glassy carbon was produced.
The preparation of glassy carbon involves subjecting the organic precursors to a series of heat treatments at temperatures up to 3000 °C. Unlike many non-graphitizing carbons, they are impermeable to gases and are chemically extremely inert, especially those prepared at very high temperatures. It has been demonstrated that the rates of oxidation of certain glassy carbons in oxygen, carbon dioxide or water vapor are lower than those of any other carbon. They are also highly resistant to attack by acids. Thus, while normal graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large ...
is reduced to a powder by a mixture of concentrated sulfuric and nitric acids at room temperature, glassy carbon is unaffected by such treatment, even after several months.
Atomic and diatomic carbon
Under certain conditions, carbon can be found in its atomic form. It can be formed by vaporizing graphite, by passing large electric currents to form acarbon arc
An arc lamp or arc light is a lamp that produces light by an electric arc (also called a voltaic arc).
The carbon arc light, which consists of an arc between carbon electrodes in air, invented by Humphry Davy in the first decade of the 1800s, ...
under very low pressures. It is extremely reactive, but it is an intermediate product used in the creation of carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
s.
Diatomic carbon
Diatomic carbon (systematically named dicarbon and 1λ2,2λ2-ethene), is a green, gaseous inorganic chemical with the chemical formula C=C (also written 2or C2). It is kinetically unstable at ambient temperature and pressure, being removed throug ...
can also be found under certain conditions. It is often detected via spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wa ...
in extraterrestrial bodies, including comet
A comet is an icy, small Solar System body that, when passing close to the Sun, warms and begins to release gases, a process that is called outgassing. This produces a visible atmosphere or coma, and sometimes also a tail. These phenomena ar ...
s and certain star
A star is an astronomical object comprising a luminous spheroid of plasma (physics), plasma held together by its gravity. The List of nearest stars and brown dwarfs, nearest star to Earth is the Sun. Many other stars are visible to the naked ...
s.
Carbon nanofoam
Carbon nanofoam is the fifth known allotrope of carbon, discovered in 1997 by Andrei V. Rode and co-workers at theAustralian National University
The Australian National University (ANU) is a public research university located in Canberra, the capital of Australia. Its main campus in Acton encompasses seven teaching and research colleges, in addition to several national academies and ...
in Canberra
Canberra ( )
is the capital city of Australia. Founded following the federation of the colonies of Australia as the seat of government for the new nation, it is Australia's largest inland city and the eighth-largest city overall. The ci ...
. It consists of a low-density cluster-assembly of carbon atoms strung together in a loose three-dimensional web.
Each cluster is about 6 nanometers wide and consists of about 4000 carbon atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s linked in graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large ...
-like sheets that are given negative curvature by the inclusion of heptagon
In geometry, a heptagon or septagon is a seven-sided polygon or 7-gon.
The heptagon is sometimes referred to as the septagon, using "sept-" (an elision of ''septua-'', a Latin-derived numerical prefix, rather than ''hepta-'', a Greek-derived num ...
s among the regular hexagon
In geometry, a hexagon (from Ancient Greek, Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple polygon, simple (non-self-intersecting) hexagon is 720°.
Regular hexa ...
al pattern. This is the opposite of what happens in the case of buckminsterfullerene
Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a soccer ball. Each of its 60 carbon atoms is bonded ...
s, in which carbon sheets are given positive curvature by the inclusion of pentagon
In geometry, a pentagon (from the Greek πέντε ''pente'' meaning ''five'' and γωνία ''gonia'' meaning ''angle'') is any five-sided polygon or 5-gon. The sum of the internal angles in a simple pentagon is 540°.
A pentagon may be simpl ...
s.
The large-scale structure of carbon nanofoam is similar to that of an aerogel
Aerogels are a class of synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas, without significant collapse of the gel structure. The result is a solid with extremely low ...
, but with 1% of the density of previously produced carbon aerogels – only a few times the density of air
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing f ...
at sea level
Mean sea level (MSL, often shortened to sea level) is an average surface level of one or more among Earth's coastal bodies of water from which heights such as elevation may be measured. The global MSL is a type of vertical datuma standardised g ...
. Unlike carbon aerogels, carbon nanofoam is a poor electrical conductor
In physics and electrical engineering, a conductor is an object or type of material that allows the flow of charge (electric current) in one or more directions. Materials made of metal are common electrical conductors. Electric current is gener ...
.
Carbide-derived carbon
Carbide-derived carbon (CDC) is a family of carbon materials with different surface geometries and carbon ordering that are produced via selective removal of metals from metal carbide precursors, such as TiC, SiC, , , etc. This synthesis is accomplished using chlorine treatment, hydrothermal synthesis, or high-temperature selective metal desorption under vacuum. Depending on the synthesis method, carbide precursor, and reaction parameters, multiple carbon allotropes can be achieved, including endohedral particles composed of predominantly amorphous carbon, carbon nanotubes, epitaxial graphene, nanocrystalline diamond, onion-like carbon, and graphitic ribbons, barrels, and horns. These structures exhibit high porosity and specific surface areas, with highly tunable pore diameters, making them promising materials for supercapacitor-based energy storage, water filtration and capacitive desalinization, catalyst support, and cytokine removal.Linear acetylenic carbon
A one-dimensional carbon polymer with the structure —(C≡C)n—.Cyclocarbons
Cyclo 8arbon (C18) was synthesised in 2019.Other possible allotropes
Many other allotropes have been hypothesized but have yet to be synthesized. *body-centred cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
structure. This phase has importance in astrophysics and deep interiors of planets like Uranus
Uranus is the seventh planet from the Sun. Its name is a reference to the Greek god of the sky, Uranus (mythology), Uranus (Caelus), who, according to Greek mythology, was the great-grandfather of Ares (Mars (mythology), Mars), grandfather ...
and Neptune
Neptune is the eighth planet from the Sun and the farthest known planet in the Solar System. It is the fourth-largest planet in the Solar System by diameter, the third-most-massive planet, and the densest giant planet. It is 17 times ...
. Various structures have been proposed. Superdense and superhard material resembling this phase was synthesized and published in 1979 and reported to have the Im space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchan ...
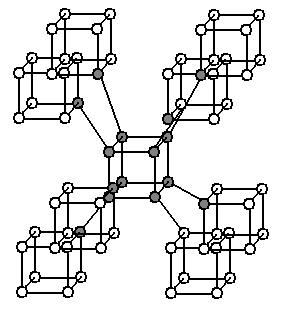
with eight atoms per primitive unit cell (16 atoms per conventional unit cell). Claims were made that the so-called C structure had been synthesized, having eight-carbon cubes similar to cubane
Cubane () is a synthetic hydrocarbon compound that consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons an ...
in the Imm space group, with eight atoms per primitive unit cell, or 16 atoms per conventional unit cell (also called supercubane, see illustration to the right). But a paper in 1988 claimed that a better theory was that the structure was the same as that of an allotrope of silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic tab ...
called Si-III or γ-silicon, the so-called BC8 structure with space group Ia and 8 atoms per primitive unit cell (16 atoms per conventional unit cell). In 2008 it was reported that the cubane-like structure had been identified. A paper in 2012 considered four proposed structures, the supercubane structure, the BC8 structure, a structure with clusters of four carbon atoms in tetrahedra in space group I3m having four atoms per primitive unit cell (eight per conventional unit cell), and a structure the authors called "carbon sodalite
Sodalite ( ) is a tectosilicate mineral with the formula , with royal blue varieties widely used as an wikt:ornamental, ornamental gemstone. Although massive sodalite samples are opaque, crystals are usually transparent to translucent. Sodalite i ...
". They found in favor of this carbon sodalite structure, with a calculated density of 2.927 g/cm, shown in the upper left of the illustration under the abstract. This structure has just six atoms per primitive unit cell (twelve per conventional unit cell). The carbon atoms are in the same locations as the silicon and aluminum atoms of the mineral sodalite. The space group, I3m, is the same as the fully expanded form of sodalite would have if sodalite had just silicon or just aluminum.
* bct-carbon: Body-centered tetragonal carbon was proposed by theorists in 2010.
* Chaoite is a mineral believed to have been formed in meteorite impacts. It has been described as slightly harder than graphite with a reflection color of grey to white. However, the existence of carbyne phases is disputed – see the article on chaoite for details.
* D-carbon: D-carbon was proposed by theorists in 2018.
D-carbon is an orthorhombic sp3 carbon allotrope (6 atoms per cell). Total-energy calculations demonstrate that D-carbon is energetically more favorable than the previously proposed T6 structure (with 6 atoms per cell) as well as many others.
*Haeckelites
Haeckelites are three-fold coordinated networks of carbon atoms generated by a periodic arrangement of pentagons, hexagons and heptagons. They were first proposed by Humberto and Mauricio Terrones and their colleagues in 2000. They were named in ho ...
: Ordered arrangements of pentagons, hexagons, and heptagons which can either be flat or tubular.
Laves graph
In geometry and crystallography, the Laves graph is an infinite and highly symmetric system of points and line segments in three-dimensional Euclidean space, forming a periodic graph. Three equal-length segments meet at 120° angles at each po ...
or ''K''4 crystal is a theoretically predicted three-dimensional crystalline metastable carbon structure in which each carbon atom is bonded to three others, at 120° angles (like graphite), but where the bond planes of adjacent layers lie at an angle of 70.5°, rather than coinciding.
* M-carbon: Monoclinic C-centered carbon is thought to have been first created in 1963 by compressing graphite at room temperature. Its structure was theorized in 2006,
then in 2009 it was related to those experimental observations.
Many structural candidates, including bct-carbon, were proposed to be equally compatible with experimental data available at the time, until in 2012 it was shown theoretically that this structure is kinetically the most likely to form from graphite.
High-resolution data appeared shortly after, demonstrating that among all structure candidates only M-carbon is compatible with experiment.
* Metallic carbon: Theoretical studies have shown that there are regions in the phase diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous ...
, at extremely high pressures, where carbon has metallic character. Laser shock experiments and theory indicate that above 600 GPa liquid carbon is metallic.
* Novamene: A combination of both hexagonal diamond and sp2 hexagons as in graphene.
* Phagraphene: Graphene-like allotrope with distorted Dirac cones.
* Prismane C8 is a theoretically predicted metastable carbon allotrope
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the ...
comprising an atomic cluster
may refer to:
Science and technology Astronomy
* Cluster (spacecraft), constellation of four European Space Agency spacecraft
* Asteroid cluster, a small asteroid family
* Cluster II (spacecraft), a European Space Agency mission to study t ...
of eight carbon atoms, with the shape of an elongated triangular bipyramid
In geometry, the elongated triangular bipyramid (or dipyramid) or triakis triangular prism is one of the Johnson solids (), convex polyhedra whose faces are regular polygons. As the name suggests, it can be constructed by elongating a triangu ...
—a six-atom triangular
A triangle is a polygon with three edges and three vertices. It is one of the basic shapes in geometry. A triangle with vertices ''A'', ''B'', and ''C'' is denoted \triangle ABC.
In Euclidean geometry, any three points, when non- collinea ...
prism
Prism usually refers to:
* Prism (optics), a transparent optical component with flat surfaces that refract light
* Prism (geometry), a kind of polyhedron
Prism may also refer to:
Science and mathematics
* Prism (geology), a type of sedimentary ...
with two more atoms above and below its bases.
* Protomene: A hexagonal crystal structure with a fully relaxed primitive cell involving 48 atoms. Out of these, 12 atoms have the potential to switch hybridization between sp2 and sp3, forming dimers.
* Q-carbon: Ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials ...
carbon was discovered in 2015.
* T-carbon: Every carbon atom in diamond is replaced with a carbon tetrahedron (hence 'T-carbon'). This was proposed by theorists in 1985.
* There is evidence that white dwarf
A white dwarf is a stellar core remnant composed mostly of electron-degenerate matter. A white dwarf is very dense: its mass is comparable to the Sun's, while its volume is comparable to the Earth's. A white dwarf's faint luminosity comes fro ...
stars have a core of crystallized carbon and oxygen nuclei. The largest of these found in the universe so far, BPM 37093
BPM 37093 (V886 Centauri) is a variable star, variable white dwarf star of the DAV, or Pulsating white dwarf, ZZ Ceti, type, with a hydrogen atmosphere and an unusually high mass of approximately 1.1 times the Sun's. It is about from Ear ...
, is located away in the constellation Centaurus
Centaurus is a bright constellation in the southern sky. One of the 88 modern constellations by area, largest constellations, Centaurus was included among the 48 constellations listed by the 2nd-century astronomer Ptolemy, and it remains one o ...
. A news release from the Harvard-Smithsonian Center for Astrophysics described the -wide stellar core as a ''diamond'', and it was named as ''Lucy'', after the Beatles' song "Lucy in the Sky With Diamonds"; however, it is more likely an exotic form of carbon. Penta-graphene
Penta-graphene is a hypothetical carbon allotrope composed entirely of carbon pentagons and resembling the Cairo pentagonal tiling. Penta-graphene was proposed in 2014 on the basis of analyses and simulations. Further calculations predicted that ...
is a predicted carbon allotrope that utilizes the Cairo pentagonal tiling.
* U carbon is predicted to consist of corrugated layers tiled with six- or 12-atom rings, linked by covalent bonds. Notably, it can be harder than steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant ty ...
, as conductive as stainless steel, highly reflective and ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials ...
, behaving as a permanent magnet
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel, ...
at temperatures up to 125 °C.
* Zayedene: A combination of linear sp carbon chains and sp3 bulk carbon. The structure of these crystalline carbon allotropes consists of sp chains inserted in cylindrical cavities periodically arranged in hexagonal diamond (lonsdaleite).
Variability of carbon
 The system of carbon allotropes spans an astounding range of extremes, considering that they are all merely structural formations of the same element.
Between diamond and graphite:
* Diamond crystallizes in the cubic system but graphite crystallizes in the hexagonal system.
* Diamond is clear and transparent, but graphite is black and opaque.
* Diamond is the hardest mineral known (10 on the
The system of carbon allotropes spans an astounding range of extremes, considering that they are all merely structural formations of the same element.
Between diamond and graphite:
* Diamond crystallizes in the cubic system but graphite crystallizes in the hexagonal system.
* Diamond is clear and transparent, but graphite is black and opaque.
* Diamond is the hardest mineral known (10 on the Mohs scale
The Mohs scale of mineral hardness () is a qualitative ordinal scale, from 1 to 10, characterizing scratch resistance of various minerals through the ability of harder material to scratch softer material.
The scale was introduced in 1812 by th ...
), but graphite is one of the softest (1–2 on Mohs scale
The Mohs scale of mineral hardness () is a qualitative ordinal scale, from 1 to 10, characterizing scratch resistance of various minerals through the ability of harder material to scratch softer material.
The scale was introduced in 1812 by th ...
).
* Diamond is the ultimate abrasive, but graphite is soft and is a very good lubricant.
* Diamond is an excellent electrical insulator, but graphite is an excellent conductor.
* Diamond is an excellent thermal conductor, but some forms of graphite are used for thermal insulation (for example heat shields and firebreaks).
* At standard temperature and pressure, graphite is the thermodynamically stable form. Thus diamonds do not exist forever. The conversion from diamond to graphite, however, has a very high activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
and is therefore extremely slow.
Despite the hardness of diamonds, the chemical bonds that hold the carbon atoms in diamonds together are actually weaker than those that hold together graphite. The difference is that in diamond, the bonds form an inflexible three-dimensional lattice. In graphite, the atoms are tightly bonded into sheets, but the sheets can slide easily over each other, making graphite soft.
See also
* Superdense carbon allotropesReferences
External links
* * * {{DEFAULTSORT:Carbon Allotropes Carbon